
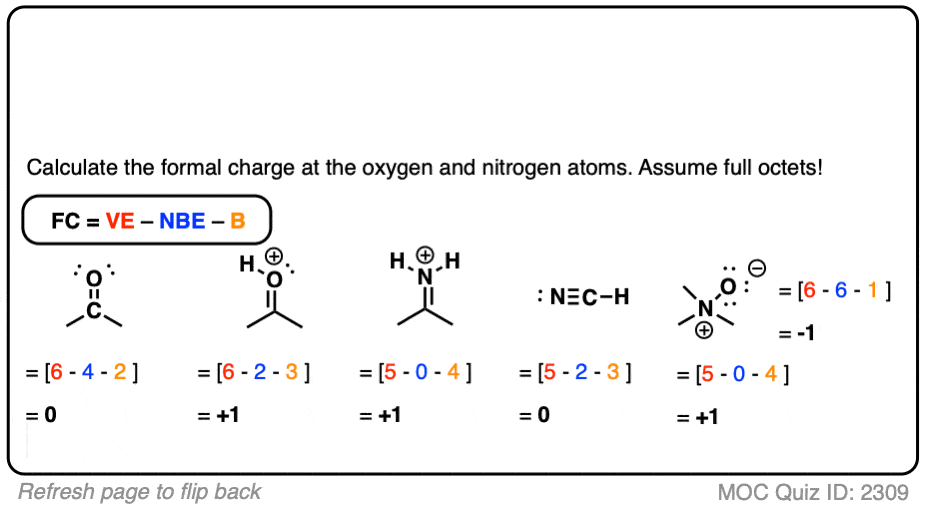

This video will explain how to find the formal charges. All that you have to do is substitute these values in the above formula. Practice drawing these lewis structures and dont worry we will go over all the answers step by step. The number of electrons in non-bonded state are 2, while the ones in the covalent bond are 8. The number of valence electrons of carbon are 4. Here, carbon is bonded with four hydrogen atoms. Formal Charge Molecules or polyatomic ions containing atoms that can. Let me now illustrate the calculation for a carbon atom in methane molecule (CH 4). Nonequlvalent Lewis structures contain different numbers of single and multiple bonds. Kossel-lewis Approach to Chemical Bonding - Formal Charge, Kossel-lewis. The right Lewis structure will be the one for which the sum of formal charges has a minimum value. Balbharati Solutions for 11th Chemistry (11th) Chapter 4: Structure of Atom. To be able to decide which is the most stable Lewis structure for any compound, one must calculate the formal charge of every atom involved in the compound and sum up all the values. In this formula, V stands for the number of valence electrons of that atom ( these are the electrons that revolve in the outermost orbit of the atom), N stands for the number of non-bonded electrons, and B stands for the number of electrons that are a part of the covalent bonds made by the atom. It is the difference between the valence electron number of the atoms and the combined sum of bonded and non-bonded electrons in an atom. Formal charge calculation enables you to determine this. This necessitates that a chemist knows the low energy electronic configuration for every one of the bonding atoms. When determining the exact molecular structure, one needs to know how the molecules have bonded together. Nature prefers low energy states which lead to stable molecular structures. DefinitionĪtoms combine together to form molecules by sharing electrons ( forming a covalent bond) or by exchanging electrons ( through an ionic bond). Another pictorial technique for depicting the electronic distribution and bonding of a molecule is a Lewis structure. Molecular nomenclatures are developed to accurately describe the structural details and expanse of any molecule. A large part of chemistry study is about learning to identify and visualize molecules. You need to be adept at experimenting and theorizing to be a chemist. 7.5 Strengths of Ionic and Covalent Bonds.

Write the Lewis structure for sulfuric acid, H 2 SO 4, which has two oxygen atoms and two OH groups bonded to the sulfur. What I like about chemistry is the amazing amount of detective work that is involved in determining the actual structure of a molecule. Write Lewis structures for each of the four compounds and determine the formal charge of the iodine atom in each molecule: (a) IF (b) IF 3 (c) IF 5 (d) IF 7.


 0 kommentar(er)
0 kommentar(er)
